Spying on Bacteria: Putting antimicrobial resistance under the spotlight
Dr Raveen Tank is a Postdoctoral Researcher working in Rok Krasovec’s group at The University of Manchester
I recently joined The University of Manchester as a Research Associate, where I use Super Resolution Microscopy to investigate DNA mutations in bacteria which can result in antimicrobial resistance.
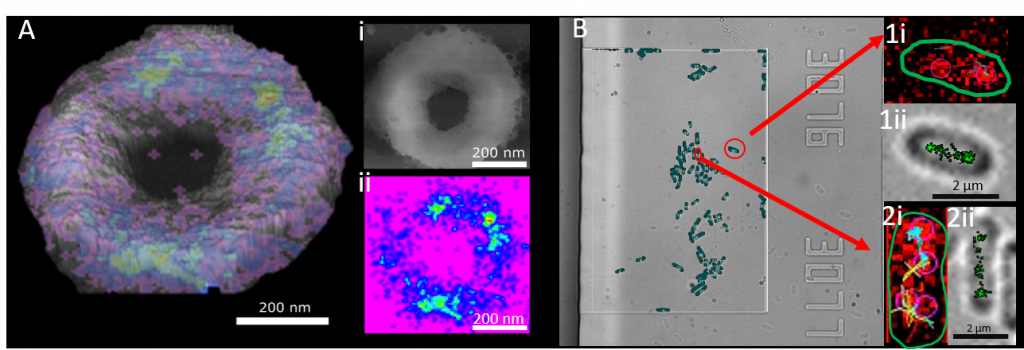
Having studied Physics at The University of Salford I did my PhD “Using STORMForce for understanding how bacteria grow and die” at The University of Sheffiled under the supervision of Professor Jamie Hobbs, Professor Simon Foster and Professor Ashley Cadby. It was like entering an entirely new world, leaving a pure physics degree where I had only touched on biophysics and being immersed into a word where our understanding of physics was used as an application to investigate biological processes. I used atomic force microscopy (AFM) and Super resolution fluorescence microscopy to investigate how bacterial cell wall is formed. Cell wall is made of a material called peptidoglycan which is the main target for antibiotics, however we still know very little about the structure of peptidoglycan and its dynamics throughout the cell cycle. Atomic force microscopy can provide near molecular resolution images of biological systems but typically provides limited chemical information. Conversely, while super-resolution optical microscopy allows localisation of particular molecules and chemistries, even to the single molecule level, information on the molecular context is difficult to obtain. These approaches were combined into STORMForce (stochastic optical reconstruction with atomic force microscopy) to map the synthesis of the bacterial cell wall structural macromolecule, peptidoglycan, during growth and division in the rod-shaped bacterium Bacillus subtilis. This technology can be used to aid our understanding of the bacterial structure and aid in our combat against antimicrobial resistance.
At Manchester I am continuing to combine my training in physics with biological questions in the Krašovec and MERMAN group where I am currently imaging DNA repair proteins within living bacterial cells. Using super resolution fluorescence optics in correlation with microfluidic devices we are able to see individual DNA repair proteins as they move around the cell. The microfluidic devices we use are really cheap as they are cast using PDMS from a silicone nitrite mould. Imaging the bacteria in this way allows for us not only to track DNA repair proteins in single cells in a more native environment but to also investigate how cell-cell interactions within a population of cells affect mutation dynamics. In the time I have done this project I have learnt so much microbiology and I have actively felt my brain being pushed out of its comfort zone, helping me see problems from a different perspective.
By pushing research in a more interdisciplinary direction we are able to combine optical physics with microbiology to visually pinpoint events of mutations which can lead to bacterial evolution and subsequently antimicrobial resistance, all in a more native environment provided by the microfluidic device. For me this has always been the beauty of interdisciplinary science. You are surrounded by people with a wealth of knowledge, coming from different backgrounds and using their combined experience to tackle problems and develop solutions coming from a different perspective. The AMR crises needs this kind of research, “many hands makes light work” and that’s never been truer than with the many minds collaborating in interdisciplinary science.

0 Comments