Who we are
The research team, led by Prof. Martin Schröder and Dr Sihai Yang, are interested in the area of materials chemistry. Specifically,
we focus on the design, synthesis and study of porous metal-organic framework (MOF) materials for energy and environmental applications.
Our group have developed a series of robust MOFs called Manchester Framework Materials (MFM). Current focus lies in the separation and capture of fuel and toxic gases, hydrocarbons and metal values, and applications of porous materials in catalysis, clean-up and proton conductivity.
A breath of fresh air
 Will we breathe fresh air again?
Will we breathe fresh air again?
The Royal Society’s Summer Science Showcase 2021 – find out more about how new porous materials are being developed to tackle challenges such as air pollution.
Join the mailing list to get the latest announcements and book tickets to the workshops.
Our research
Our research themes cover a wide range of interests:
- Energy storage
- Proton conductivity
- Toxic gas separation
- Biomass conversion
- Radioactive iodine adsorption
Overview
We are interested in developing solid materials for the applications in clean-air technology, catalysis, biomass utilisation, energy storage, separation and conductivity. We study a wide range of porous materials based upon metal-organic frameworks, zeolites, and inorganic materials. Our major interest is to investigate the key chemical processes involved in host-guest binding underpinning their materials function, using in situ structural and dynamic studies by synchrotron X-ray diffraction, spectroscopy and neutron scattering, combined with computational modelling. We have frequent access to National synchrotron and neutron facilities worldwide.
Porous materials containing nanosized cavities (1-20 nm), the walls of which are decorated with various active sites, can form unique functional platforms to study and re-define the chemistry of small molecules within confined space. Research in the group involves design, synthesis and characterisation of the materials, and more importantly the structural and dynamic studies at national facilities to understand their material functions at a molecular level. Recent finding includes the discovery of catalytic origins and reaction intermediates for a range of important biomass catalytic conversions, and a series of new metal-organic frameworks showing exciting properties for the clean-up of air pollutants.
Energy storage
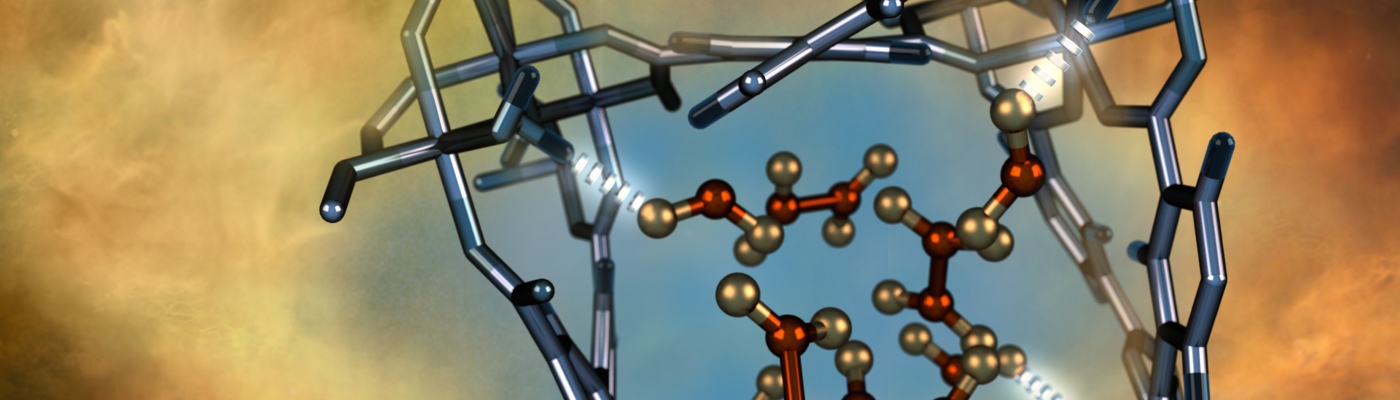
 Alternative fuels such as ammonia, hydrogen and methane have great potential as energy resources but require safe and efficient storage solutions.
Alternative fuels such as ammonia, hydrogen and methane have great potential as energy resources but require safe and efficient storage solutions.
We research MOFs with high storage capacities and packing densities for these gases. (Angew. Chem. Int. Ed., 2018, 57, 14778 –14781; Inorg. Chem., 2018, 57, 12050−12055; J. Am. Chem. Soc., 2016, 138, 29, 9119-9127)
Proton conductivity
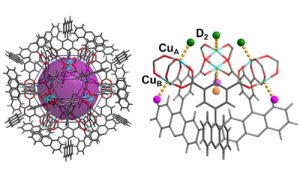
 We are interested in developing MOFs with high proton conductivity as proton exchange membrane for fuel cell applications.
We are interested in developing MOFs with high proton conductivity as proton exchange membrane for fuel cell applications.
We also investigate the proton conducting mechanism of applied MOFs by using structural and dynamic studies such as X-ray diffraction, neutron scattering, 2H NMR as well as computational modelling. (J. Am. Chem. Soc., 2016, 138, 6352−6355; Chem. Mater., 2018, 30, 7593−7602; Chem. Sci., 2019, 10, 1492.)
Toxic gas separation
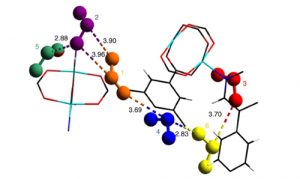 We are interested in investigating chemically stable MOFs with high uptake capacities and selectivity for the adsorption of corrosive toxic gases such as SO2 and NO2.
We are interested in investigating chemically stable MOFs with high uptake capacities and selectivity for the adsorption of corrosive toxic gases such as SO2 and NO2.
We have recently reported a number of MOFs which have demonstrated excellent separation performances of these species from simulated exhaust gas mixtures. (Nat. Chem. 2019, 11, 1085–1090; Nat. Mater. 2019, 18, 1358−1365; Nat. Mater. 2018, 17, 691−696)
Biomass conversion
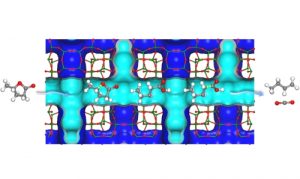 We are interested in developing zeolites with high catalytic performance for biomass conversion. Our current research areas include catalytic upgrading of biomass-derived sugars and platform molecules, depolymerisation and hydrodeoxygenation of lignin.
We are interested in developing zeolites with high catalytic performance for biomass conversion. Our current research areas include catalytic upgrading of biomass-derived sugars and platform molecules, depolymerisation and hydrodeoxygenation of lignin.
We also investigate reaction mechanisms at atomic-level by using structural and dynamic studies such as X-ray diffraction, neutron scattering, X-ray adsorption as well as computational modelling. (Nat. Mater. 2020, 19, 86–93. Nat. Commun. 2017, 8, 16104. Nat. Commun. 2016, 7, 11162. Chem 2019, 5, 1521-1536.)
Radioactive iodine adsorption
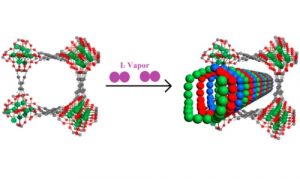 Radioactive iodine produced during the generation of nuclear power poses a risk to human health if it is allowed to escape into the environment. Radioactive iodine is particularly harmful due to its ability to spread quickly over a large area and remain radioactive long periods of time.
Radioactive iodine produced during the generation of nuclear power poses a risk to human health if it is allowed to escape into the environment. Radioactive iodine is particularly harmful due to its ability to spread quickly over a large area and remain radioactive long periods of time.
We have demonstrated that MFM-300 performs well for the capture of iodine due to its high stability and the confinement effect of iodine molecules into triple-helical chains. (Inorg. Chem. 2019, 58, 20, 14145-14150, J. Am. Chem. Soc. 2017, 139, 45, 16289-16296)
Publications
Discover more about our research advances in our publications:

Our team
The research group is led by Prof. Martin Schröder and Dr Sihai Yang and features an international team of interdisciplinary researchers based in the School of Chemistry at the University of Manchester.
