Dr Jean-Michel Fustin
 Jean-Michel talks about his lab and research into methyl metabolism and the biological clock.
Jean-Michel talks about his lab and research into methyl metabolism and the biological clock.
What does your lab research?
At the Meth Lab, we focus on methyl metabolism, and more precisely how methyl metabolism interacts with our biological clock.
Biological methylation of nucleic acids, proteins, fatty acids and small molecules are catalysed by methyltransferases that use the methyl donor co-substrate S-adenosylmethionine (SAM), synthesised by a universal metabolic pathway called the methyl cycle. The synthesis of SAM requires the essential nutrients methionine, vitamin B9, B12 and choline.
While the role of histone methylation in the function of our biological clock is relatively well understood, there are many more methylations whose physiological roles are uncertain, including RNA methylation and non-histone protein methylation.
We study the function of selected methylated nucleotides in messenger RNA, and we seek to define how SAM availability regulates general cellular methylations.
We use molecular and behavioural circadian rhythms, which depend on transcription-translation feedback loops, as a read-out for the effects of genetic, dietary and pharmacological methylation deficiencies.
How did you originally become a researcher in this area?
I’ve always loved bugs and monsters, so biology was an easy choice. I originally wanted to teach science in secondary schools, so after graduating from The University of Namur in Belgium, I obtained a postgraduate degree in education.
As a part of this degree, I gave a series of courses in the school I myself studied at. While I enjoyed this tremendously, I felt that not knowing what research actually is was hampering my ability to teach science.
At the same time, I felt too limited by my environment in Belgium. I first took a job as a research assistant in Scotland (Aberdeen), where I met Dr David Hazlerigg and his team who motivated me to continue my career in academia.
In this job, I was involved with the study of the genetic bases of obesity. I then did a PhD on seasonal rhythms, supervised by Prof Hazlerigg.
How did you come to set up the Meth Lab in Manchester?
I moved to Kyoto in Japan to work with Prof Hitoshi Okamura for my postdoc, where I eventually became an associate professor and founded my own Laboratory of Molecular Metabology.
In 2013, we reported that the N6-methylation of internal adenosines in mRNA (m6A) was a regulator of circadian rhythms, which was the first physiological function demonstrated for m6A.
We next reported that the expression of Casein Kinase 1 Delta (CK1D), a critical kinase in the control of circadian rhythms, cellular growth, and survival, is regulated at the posttranscriptional level by m6A.
In collaboration with DukeNUS, we discovered that two alternatively spliced isoforms of CK1D had antagonistic functions in the control of circadian rhythms.
In 2019, I was awarded a Future Leaders Fellowship to relocate my lab to the University of Manchester, where it was renamed to the Meth Lab.
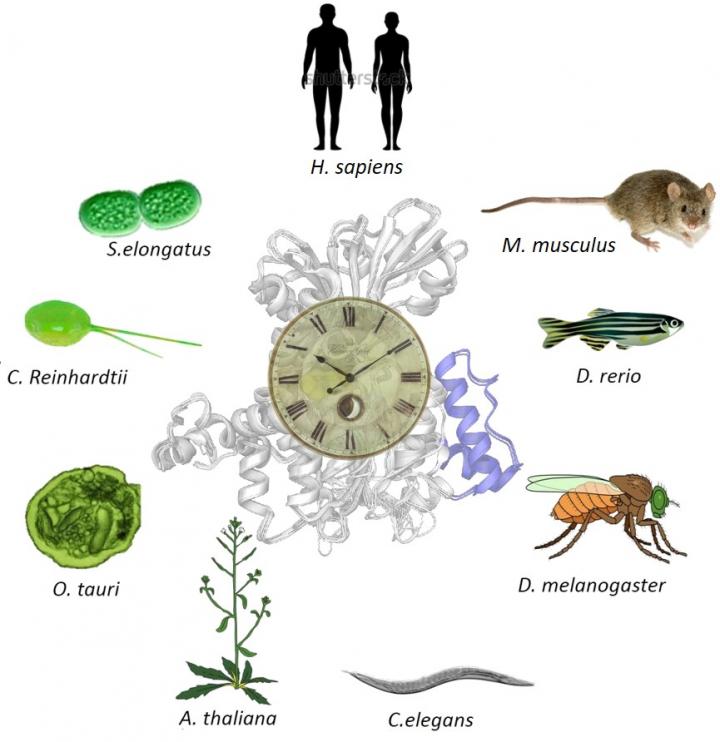
In a major intercontinental collaborative effort in 2020, we then reported that the inhibition of methyl metabolism had nearly identical effects on biological rhythms in bacteria, unicellular green algae, plants, invertebrates, fish and mammals, further underlining the intimate relationship between biological rhythms and methyl metabolism.
What has been your favourite outreach activity and what made it stand out?
Various lectures about my research at secondary schools in Japan. To be able to connect with young students, some of whom may be the next generation of researchers, was very rewarding.
These lectures were not the subject of subsequent exams, and I felt it was liberating for both the students and I. I could depart from the usually strict curriculum that students have, and the students could study something that is not usually included in their science courses, something concrete and current, something being researched at universities now.
My hope is that I will be able to organise such activities here in the UK.
What are your top three key publications so far?
Excess S-Adenosylmethionine inhibits methylation via catabolism to adenine
S-adenosylmethionine (SAM), the endogenous cosubstrate in methyl group transfers, is widely available as a dietary supplement.
Against expectations, in this new manuscript we found exogenous SAM is catabolized to adenine, an inhibitor of adenosylhomocysteinase, leading to widespread methylation inhibition and disruption of circadian rhythms in vitro and in mice.
DOI: 10.21203/rs.3.rs-934744/v1
Methylation deficiency disrupts biological rhythms from bacteria to humans
The methyl cycle is a universal metabolic pathway providing methyl groups for the methylation of nuclei acids and proteins, regulating all aspects of cellular physiology.
In this paper, we have shown that methyl cycle inhibition affects biological rhythms in species ranging from unicellular algae to humans, separated by more than 1 billion years of evolution.
DOI: 10.1038/s42003-020-0942-0
RNA-methylation-dependent RNA processing controls the speed of the circadian clock.
The eukaryotic biological clock involves a negative transcription-translation feedback loop in which clock genes regulate their own transcription and that of output genes of metabolic significance.
In this paper, we have reported that inhibition of the methyl cycle affects biological rhythms in mouse and human cells, and in living mice. We identified the methylation of messenger RNAs as a major mechanism linking biological rhythms with methyl metabolism.
DOI: 10.1016/j.cell.2013.10.026
See a list of Jean-Michel’s publications in Research Explorer.
Lab members
- Ben Saer (Senior Research Technician)
Collaborators
- Prof Hitoshi Okamura, University of Kyoto
- Prof Alastair Stewart, University of Melbourne
- Prof Victoria Cowling, University of Dundee
- Prof Hugh Piggins, University of Bristol
- Dr Chao Hsu-Wen, Taipei Medical University
More about Jean-Michel
- Read Jean-Michel’s research profile in Research Explorer.
- Visit The Meth Lab website.






0 Comments