
News
January 2024: Latest ConnecteDNA Newsletter
Please find our latest newsletter here: Newsletter
October 2023: New Publication in the RBMO
Lucy Frith was published alongside other colleagues in Reproductive BioMedicine Online in a paper titled ‘Direct-to-consumer genetic testing and the changing landscape of gamete donor conception: key issues for practitioners and stakeholders’. Find all the information, and the open access paper here.
October 2023. Opening the Register 2023: preparing for contact
The law changed in 2005 to enable donor-conceived people, at age 18, to access information about their gamete or embryo donor. The first cohort of people entitled to access this information were able to do so from October, 2023 through the HFEA’s ‘Opening the register’ programme.
Researchers from The ConnecteDNA project have (with the support of a graphic designer) led the design and creation of a series of leaflets to help donors and their families prepare for the possibility of contact. You can read our leaflets on the ‘Preparing for Contact’ page.
This work was funded by a UKRI ESRC Impact Accelerator Award.
September, 2023
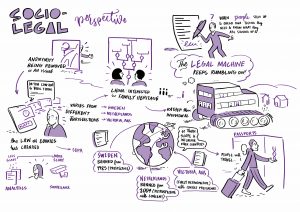
29 September: Caroline was invited to speak at the 2023 Symposium of the Association of Reproductive & Clinical Scientists. Her talk was entitled, ‘A socio-legal perspective on the experiences of donor conception and direct-to-consumer genetic testing: what is the role of law?
14 September: Caroline presented a paper at the EACME Conference 2023, held at The University of Warsaw. Caroline’s paper was entitled, ‘Donor anonymity and Justice’.
June and July, 2023. Meetings with members of the APPG on health.
In June and July, 2023, Lucy Frith and Caroline Redhead, working with Policy@Manchester, had two online meetings with members of the APPG on Health to discuss a ConnectedNA policy brief relating to the implications of technology on gamete donor anonymity. You can read our policy recommendations in our Policy@Manchester blog post, ConnecteDNA – the implications of technology on donor conception anonymity. The blog is available here: https://research.manchester.ac.uk/en/clippings/connectedna-the-implications-of-technology-on-donor-conception-an
June 2023.
28 June: Caroline presented a paper entitled, ‘Datafied DNA and donor-conception’ at a one-day online conference hosted by the University of Surrey, The Datafied Family: Algorithmic Encounters in Care, Intimacies, Routine and Play.
13 June: New collaboration with researchers from the University of Oulu, Finland.
Lucy, Caroline and Leah hosted three Finnish researchers at Manchester in June 2023. We held a very productive workshop at which we all presented our work, and then discussed the focus of the new collaboration. We are planning to develop a collaborative network. Read more about it here: https://www.oulu.fi/en/research-groups/dnadialogues [oulu.fi]
May 2023
22 May: Caroline and Lucy published a Policy@Manchester blog discussing the implications of technology on gamete (egg, sperm and embryo) donor anonymity.
You can read our blog here: https://blog.policy.manchester.ac.uk/posts/2023/05/connectedna-the-implications-of-technology-on-donor-conception-anonymity/
April, 2023.
3 April: PET BioNews published Caroline’s article on donor anonymity, which reflected some of the preliminary findings of the stakeholder workshops and the qualitative interviews. It also discussed some of the issues highlighted by the ongoing HFEA Consultation.
You can read the HFEA Consultation here: https://www.hfea.gov.uk/about-us/modernising-the-regulation-of-fertility-treatment-and-research-involving-human-embryos
You can read our BioNews article here: https://www.progress.org.uk/the-connectedna-project-thinking-about-law-reform-and-gamete-donor-anonymity/
4 April 2023: Caroline presented a paper, ‘What’s the right age to know (about) your donor? Exploring age, agency, best interests and children’s decision-making capacity in relation to DNA testing and donor conception’ at the Socio-Legal Studies Association Annual Conference, held at the University of Ulster, Magee Campus, Derry/Londonderry.
12 April 2023: Leah presented a paper, ‘What’s the right age to know your donor? Exploring children’s rights, agency and kinship through decisions about DNA testing and donor conception’ at the British Sociological Association Annual Conference, held at The University of Manchester.
14 April: We submitted our response to the HFEA consultation on reforming the HFE Act.
Our responses to the HFEA’s questions about donor anonymity, based on our key preliminary findings, are summarised briefly below. You can read the full response here: [see word document attached to the email].
Clinics should be required by law to inform donors and recipients of the potential for donor identity to be discovered through DNA testing websites [Q21]
We support the aim of this proposal to ensure that donors and recipients are properly informed about the potential for donors’ identity to be discovered as a result of the operation of emerging technologies such as DTCGT. However, in our view, making specific reference to this exact technology in primary legislation would not ‘future-proof’ the Act. There are other emerging technologies, such as facial recognition technologies to identify genetic relatives, which are likely to have similar impacts (and that may have further implications which are as yet unclear). Thus, reference to specific technologies might best be contained in Guidance/the HFEA Code of Practice, rather than in primary legislation.
The Act should be amended to provide parental and donor choice to opt for anonymity until age 18 (as now) or identifiable information on request after the birth of a child [Q22]
While we agree that the ‘dual track’ approach suggested would be preferable to the current legal position, our data suggest that an agree/disagree response is insufficiently nuanced. A more flexible system would therefore be preferable to a ‘dual track’ system. Further, interviews with regulators in the Netherlands (where there was a ‘dual track’ system until 2004) highlighted the importance of counselling donors about the implications and limitations of their decisions around identifiability, and ensuring that donors cannot legally withdraw consent to be identifiable once they have donated gametes on the basis of such consent. We also raised a number of concerns about the potential for inequalities to be created / exacerbated by a ‘dual track’ system (including donor conceived people, possibly within the same family, having different ‘rights’) and reflected our participants’ concern that, in any system in which donor anonymity remains a possibility, the paramountcy of the donor-conceived child’s welfare is undermined.
We also suggested, even if donor anonymity is retained until a donor-conceived child turns 18, connections with same-donor siblings are often seen as equally important, and sometimes more important, to donor conceived people than contact with the donor. In the absence of legal routes to access information about same-donor siblings during the childhood of a donor conceived person, parents often turn to online DNA testing. We suggested that opening up the Donor Sibling Link (DSL) to parents and donor conceived people at a younger age would avoid them unnecessarily undertaking the various social risks involved in using DTCGT.
The Act should require all donors and recipients to have access to information about the implications of their decision before starting treatment [Q23]
In our response, we noted that question 23 asks whether the Act should require all donors and recipients to have implications counselling before starting treatment. We suggest that terminology and language are important here, particularly in the context of a proposal that ‘counselling,’ which is always understood to be offered rather than imposed, be mandated. ‘Counselling’ might not be an appropriate term for mandated meetings where information-sharing is the core purpose.
Subject to our concern about terminology, we supported the proposal that implications counselling be mandatory. We recommended that the Act require donors and recipients to have a minimum of two (funded) implications counselling sessions, with some reflection time in between. We also suggested that the Act should require that such counselling be offered by qualified counsellors (ideally accredited by the British Infertility Counselling Association).
Further, we note the importance of implications counselling being attentive to the potential impact on the relatives of donors and donor conceived people by the sharing of information about donor conception through DTCGT.
15 April 2023: Lucy presented a paper, ‘Direct to consumer DNA Testing and Donor Conception’ at the Donor Conception Network Spring Conference 2023, held in London.
26 April 2023: Leah was invited to present a paper, ‘Bypassing the Official Record: Exploring the Social and Ethical Issues Raised by Direct-to-Consumer Genetic Databases for Donor Conceived Children and their Parents’ to Liverpool University Archive Studies Seminar Series
February 2023
On 28th February, 2023, the HFEA published a public consultation about modernising the regulation of fertility treatment and research involving human embryos. The consultation was split into four areas where the HFEA considered modernisation is most needed. These were:
• Patient safety and promoting good practice
• Access to donation information
• Consent
• Scientific developments
You can read the press release here: https://www.hfea.gov.uk/about-us/news-and-press-releases/2023-news-and-press-releases/fertility-law-needs-modernising-says-uk-regulator/
You can read the consultation questions here:
https://www.hfea.gov.uk/about-us/modernising-the-regulation-of-fertility-treatment-and-research-involving-human-embryos/
The ConnecteDNA team submitted a response to the HFEA Consultation, focusing specifically on access to donation information. You can read our response here.
November 2022 and January 2023
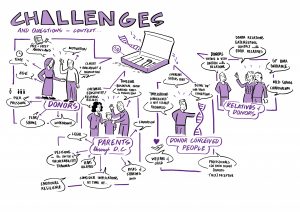
CONNECTEDNA STAKEHOLDER WORKSHOPS
We held three stakeholder workshops in Manchester (1.11.22), London (29.11.22) and Birmingham (25.1.23). The participants (40 in total – some people occupying more than one role) were donor conceived people (9), parents of donor-conceived people (10), egg and sperm donors (7) and professionals (23). 
(Slide also attached here)
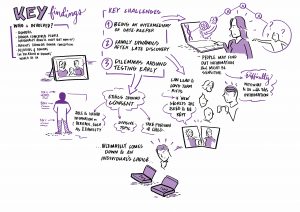
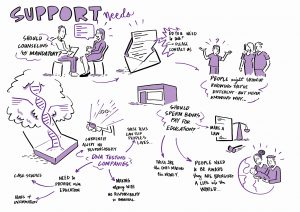
At the workshops, the focus was on discussion. To set the scene, Leah and Caroline first presented the preliminary findings of the qualitative interviews and the legal work. For the remainder of the day participants and members of the ConnecteDNA worked through group activities. We discussed the type of support people affected by donor conception might find useful, and when, taking a ‘life course’ approach, that support would be most helpful. We considered how support might be delivered – and how resources might be made available.
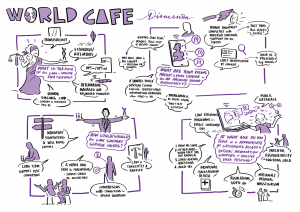
We had a legal ‘world café’ discussion, touching on the role of law in direct to consumer genetic testing and in terms of the regulation of donor anonymity, on law/policy reform in the area of donor anonymity. Our discussion questions were drawn from the HFEA’s Legislative Reform Advisory Group discussion document – available here
Our discussion questions are summarised in the slide below and attached here
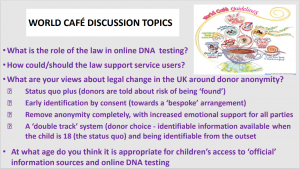
Our Birmingham workshop was attended by a graphic facilitator – Each of his scribings is linked in a large format below, and attached in preview.
1.  2.
2. 
3. 4.
4.
5. 6.
6. 
December 2022
Our second newsletter came out in December 2022. You can read a copy here.
September 2022
On 22nd September, Leah was invited to make a presentation at the ANR ‘Origines’ University, Carry-le-Rouet, France. She spoke about ‘Individualised vs. relational constructions of origins: Comparing UK policy with lived experiences of donor conception’.
July 2022
July 4th: Bionews published Leah’s article: ‘Widening the debate about direct-to-consumer genetic testing and donor conception,’ at this link: https://www.progress.org.uk/widening-the-debate-about-direct-to-consumer-genetic-testing-and-donor-conception/
July 4th: The Conversation published our article: ‘Eggs and sperm can now be stored for up to 55 years – here’s what that means for donors and people seeking fertility treatment,’ at this link:
https://theconversation.com/eggs-and-sperm-can-now-be-stored-for-up-to-55-years-heres-what-that-means-for-donors-and-people-seeking-fertility-treatment-186087
3-6 July: The ConnecteDNA project featured twice at the 2023 European Society of Human Reproduction and Embryology (ESHRE) annual conference in Milan, Italy
A press release introduced the ConnecteDNA research https://www.eshre.eu/ESHRE2022/Media/2022-Press-releases/Frith
And, on 5th July, Lucy presented our paper, ‘The implications of direct-to-consumer genetic testing for professionals and people involved in donor conception’.
20-22nd July: Lucy presented our paper, ‘What are the ethical and legal issues raised by direct to consumer genetic testing for gamete donor conception?’ at the World Congress of Bioethics, held at the University of Basel, Switzerland.
June 2022
June 15-17th Leah presented a paper: Direct-to-consumer genetic testing and donor conception: Reconfiguring relatedness in the digital age? at the REPRODUCTIVE FUTURES conference: Emergent Injustices, Hopes and Paradoxes at Tampere University, Finland
May 2022
We published our first Newsletter this month. You can read it here.
11.5.22: Lucy, Leah and Caroline gave a presentation to the Centre for Social Research and Policy (here at the UoM) as part of their Seminar Series. Our presentation was called: ‘ConnecteDNA: donor conception in the age of direct to consumer genetic testing’ Centre for Social Ethics and Policy Seminar Series, The University of Manchester.
15 May 2022: Leah presented a paper entitled; ‘Is direct-to-consumer genetic testing reconfiguring relatedness in donor-assisted reproduction?’ to the BSA Human Reproduction Study Group Conference, De Montfort University.
April 2022
Our presentation to the Socio-Legal Studies Association 2022 Annual Conference
Dr Caroline Redhead presented our paper, ‘Theories of relativity: donor conception in the age of direct to consumer genetic testing’ at the ‘Exploring Legal Borderlands’ panel at the SLSA annual conference last week.
The focus of the panel was the uncertainty and interactivity of legal borderlands, including the division between the formal and informal, law and non-law, and jurisdictional boundaries. Recognising that legal borders are often not clearly defined or static, examination of social practices falling within grey areas was encouraged, as well as a focus on the development of norms and concepts within or across legal borderlands. Looking at legal borderlands in the context of direct-to-consumer genetic testing (DTCGT) and donor conception, our presentation discussed:
- the borderland between tight regulation of assisted conception and the relative chaos of DTCGT;
- the borderland between different legal jurisdictions – DTCGT does not stop at national borders;
- how (if at all) borderlands between jurisdictions matter with the global reach of both donor conception and DTCGT; and
- the borderland between medicine and commerce: how do / should we regulate DTCGT?
Our presentation touched on the significance of genetic identity, considering the legal treatment of donor anonymity and comparing the legislative approach in different parts of the world. We also questioned the sufficiency of existing legal approaches to consent in the context of DTCGT, suggesting that the relational implications of genetic data need to be considered. New legal approaches to the challenges posed by digital technologies, such as dynamic consent, data stewardship, and relational theories of privacy offer food for thought. As does a movement away from the primacy of individual autonomy in the human rights context, opening the door for consideration of the rights of donor-conceived people as a distinct group.
We concluded by suggesting that;
- Important legal issues fall between the cracks in the various legal borderlands surrounding DTCGT in the particular context of donor-conception (and, arguably, generally as well);
- An internationally coherent approach to regulating assisted reproduction, and, in particular, disclosure of information about genetic identity, is urgently required; and
- A review of our understandings of consent in the context of DTCGT, which sits legal borderland between medicine and commerce, is overdue and urgently required.
March 2022 [following move to Manchester]
The ConnecteDNA research informed the design of two lectures to LLM students on The University of Manchester’s Global Health Law and Bioethics Course.
- Leah delivered a lecture on Cross-border reproduction and the global bioeconomy
- Caroline and Lucy delivered a lecture on Regulating Reproductive Technologies
February 2022
In February 2022 Lucy, Leah, Caroline and the ConnecteDNA project moved to The University of Manchester. The new home of ConnecteDNA is the Centre for Social Ethics and Policy, in the Department of Law. Follow us on Twitter @ConnecteDNA
August 2021
ConnecteDNA ideas for law reform – our suggestions to the Law Commission
The Law Commission is the statutory independent body created to keep the law of England and Wales under review, and to recommend reform where it is needed. The aim of the Commission is to ensure that the law is fair, modern, simple and cost effective.
Every few years, the Law Commission undertakes a public consultation with a view to submitting a draft programme of law reform to the Lord Chancellor. The 13th Programme was agreed in 2017 and the Commissioners has recently consulted with a view to agreeing the 14th Programme. See the Law Commission’s suggested ideas for law reform on the Law Commission website.
The ConnecteDNA team was excited to see that, in the area of family law, Law Commission is interested in hearing about ideas for reform relating to assisted conception, and access to information about people’s origins. We have responded to the consultation and told the Law Commission about our research. You can download and read a summary of our response below:
Response to Law Commission Consultation on 14th Programme (PDF)
The final programme of suggested reforms is expected to be published during the first half of 2022. We hope that our suggestions will feature.
