How the immune system contributes to Long Covid
In the next entry in our ‘Study in Focus’ series Dr Elizabeth Mann and Dr Nicholas Scott write about their study exploring the lingering effects of SARS-CoV-2 infection in patients experiencing Long COVID symptoms. Their research has uncovered unique immune signatures that define distinct subgroups of Long COVID patients, offering promising targets for the development of new drugs.
The start of COVID-19 research at the Lydia Becker Institute
At the start of the first wave of COVID-19 in the UK in March 2020, we took part in a huge collaborative effort involving many Lydia Becker Institute immunologists, clinician scientists, and research nurses across four different hospitals within Manchester to carry out widescale immune profiling of hospitalised COVID-19 patients. Our study was published in Science Immunology in October 2020, and alongside other rapid research efforts across the world, detailed distinct changes in host immunity during SARS-CoV-2 infection. We surveyed key alterations in myeloid cells of the innate immune system corresponding to disease severity, incidence of intensive care admission, and death.
This project encompassed large-scale collaborations to enable the use of fresh blood samples in real-time, across multiple time points from admission through to intensive care. Arguably one of the most striking findings of this work was the distinct changes to circulating monocytes that tracked with disease trajectory, including high monocyte expression of cell cycle marker Ki67 (also seen in Ebola and H1N1 pandemic flu) and low expression of the prostaglandin-generating enzyme COX-2.
The key collaborations made during this study, led by Professor Tracy Hussell, across lab groups within the Lydia Becker Institute and with clinical academics within Respiratory Medicine at Manchester hospitals, have enabled us to continue to study COVID-19 patients following hospital discharge during convalescence to assess how long immune responses remain abnormal.
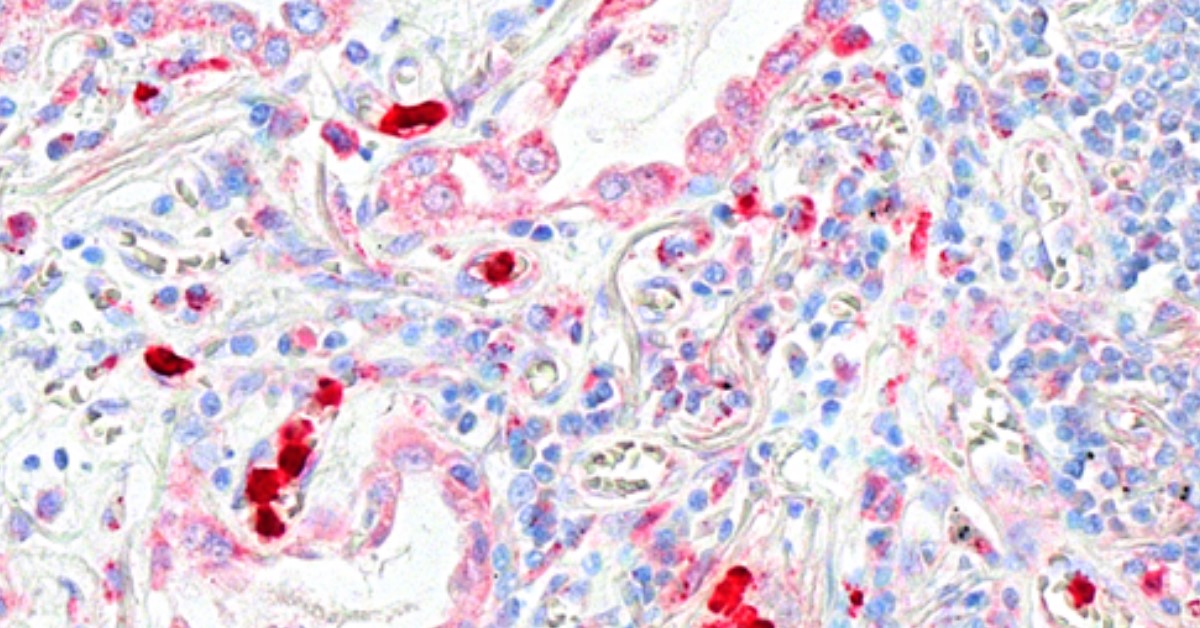
PFILD Lung (CXCL16) Image: Dr Elzabeth Mann and Dr Nicholas Scott
Our Long COVID study
Our lab has a focus on tissue-specific regulation of primary monocyte and macrophage functions, having started up in Manchester in 2017. It therefore seemed a natural progression to follow up from our acute COVID-19 study to investigate how long monocytes remained dysregulatedfollowing SARS-CoV-2 infection, and what the implications are for any persisting problems patients have during convalescence. Towards the end of 2020 it became apparent that many COVID-19 patients had long-lasting symptoms including extreme fatigue, shortness of breath, brain fog, and depression, alongside evidence of fibrotic lung disease and pulmonary vascular disease (long COVID).
To date, there are still limited treatment options for long COVID despite it remaining a global public health issue even with milder strains of SARS-CoV-2 and following worldwide vaccination programmes. This is predominantly due to the fact that the development of treatments requires an in-depth understanding of the underlying immune dysfunction.
We therefore focused on addressing whether monocytes were abnormal during recovery from COVID-19 and whether this corresponded to symptoms of long COVID. We found that a chemokine receptor that facilitates immune cell migration, CXCR6, was heightened on monocytes in acute COVID-19 whilst patients were still in hospital. Once patients had been discharged and were recovering, expression of CXCR6 remained abnormally high, but only in patients with sustained lung injury and long COVID symptoms of shortness of breath.
With the CXCR6 ligand CXCL16 expressed at high levels in the lung, we thought that this may reflect enhanced monocyte recruitment to the lung. Indeed, we set up in vitro experiments to assess the migratory capacity of monocytes from long COVID patients who were short of breath, and these monocytes preferentially migrated towards CXCL16 in experimental migration assays.
The other major symptom of long COVID is sustained fatigue, an ongoing issue for many COVID-19 patients following the acute/hospitalisation phase. With a very different immune profile to long COVID-19 patients that were short of breath, fatigued long COVID patients had monocytes that were abnormal by their reduced expression of the prostaglandin generating enzyme COX-2, alongside reduced expression of chemokine receptor CXCR2.
Intriguingly, monocytes isolated from convalescent patients suffering continued shortness of breath produced significantly less of the inflammatory cytokine tumour necrosis factor alpha (TNFa) than asymptomatic patients, and those who suffered from fatigue only, suggesting TNFa could be required for restoration of lung injury.
These unique monocyte signatures therefore defined distinct subgroups of long COVID patients, indicating key roles for monocyte migration in COVID-19 pathology and providing unique targets for the development of new drugs for long COVID

Dr Nicholas Scott at work in the Lydia Becker Institute labs Photo: Sara Kirkham
What is next?
The collaborations made during this study and the findings themselves have led to exciting new avenues of translational research for our lab, in long COVID and other lung conditions. We are now investigating the role of the CXCR6-CXCL16 axis and innate immune dysfunction in interstitial lung disease, in conjunction with our clinical academic colleagues within Respiratory Medicine.
In addition, we have been able to translate our previous murine studies demonstrating striking effects of antibiotic use on pulmonary immunity to human studies, by teaming up with clinical academics from the Respiratory Medicine team.
Read the paper published in the European Respiratory Journal

Dr Elizabeth Mann – Wellcome Trust and Royal Society Sir Henry Dale Fellow, Lydia Becker Institute of Immunology and Inflammation, University of Manchester

Dr Nicholas Scott – Postdoctoral Research Associate, Lydia Becker Institute of Immunology and Inflammation, University of Manchester





0 Comments